Aims and objectives
Imagio™ (Seno Medical Instruments,
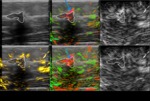
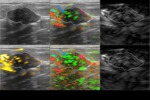
Inc.) is currently an investigational medical device being tested for FDA review. Imagio is a combination of opto-acoustics (OA) co-registered with ultrasound currently undergoing a clinical study to evaluate effectiveness. Imagio uses dual wavelength laser opto-acoustic imaging technology co-registered with conventional diagnostic ultrasound to gain both structural and functional imaging information of potentially suspicious breast masses without having to administer radioactive contrast agents or expose patients to radiation.
This hybrid imaging technology has been previously introduced in abstracts and presentations at...
Methods and materials
A prospective series of 66 cases included 37 histologically proven cancers and 29 histologically proven benign masses.
Each patient had five specific features assessed by Imagio using a 0-5 ordinal scale.
The internal tumor features included density of vascularity (DV),
blood oxygen saturation (BO),
and the total blood accumulation (BA) while external tumor features included total blood (TB) and peri-tumoral radiating vessels (RV); each were scored on a 0-5 ordinal scale and were summed to get a total internal score,
total external score,
and a...
Results
The study population consisted of 29 fibroadenomas (FAs) as well as 10 IDC 1,
11 IDC 2,
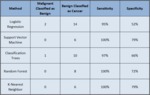
and 18 IDC 3 cases; 66 cases were evaluated for the classification analyses. Results were consistent and favorable for Imagio; there was 100% sensitivity with 72% to 79% specificity (Table 1). KNN and SVM performed the best while LR performed the worst. Findings support the stand-alone biopsy-sparing potential for Imagio.
In addition,
the probability of malignancy for these methods decreased depending on the variables with negative coefficients and...
Conclusion
Preliminary data suggest that Imagio has the ability to achieve stand-alone clinically meaningful sensitivity and specificity in a diagnostic setting.
The internal features are highly predictive consistent with the design of the co-registered methodologies. These features each carry prognosis.
Prospective research to evaluate this technology is currently underway for regulatory review when it is complete.
References
1.
Pamela M.
Otto,
Kenneth Kist,
et al.Feasibility of Co-registered Opto-Acoustic and Ultrasonic Imaging for Differentiation of Malignant From Benign Breast Tumors.Presented at:American Institute for Ultrasound in Medicine; 2012 Mar 31; Phoenix,
AZ.
2.Pamela M.
Otto,
Kenneth Kist,
et al.Clinical Feasibility Study of Combined Opto-Acoustic and Ultrasonic Imaging Modality Providing Co-registered Functional and Anatomical Maps of Breast Tumors.Presented at:Radiological Society of North America; 2011 Nov 28; Chicago,
IL.