Learning objectives
Describe the clinical background of immunotherapy in cancer treatment.
Outline the novel patterns of treatment response observed in patients receiving immunotherapy and discuss the immune-related response criteria (irRC).
Recognize the imaging findings of immune mediated adverse events.
Background
Cancer Immunotherapy refers to any form of cancer treatment that uses the immune system to target cancer cells.
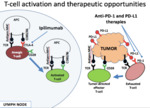
Active immunotherapy using checkpoint inhibitors is an emerging form of cancer treatment which uses monoclonal antibodies to stimulate anti-tumor T-cell activity.
Ipilimumab is a cytotoxic T-lymphocyte antigen-4 (CTLA4) checkpoint inhibitor.
CTLA4 blocks T-Cell activation when it binds to B7.
Ipilimumab,
which binds to CTLA4,
prevents this interaction,
thus stopping the downregulation of the T-cell immune system.
Another immune checkpoint that can be targeted is the programmed cell...
Findings and procedure details
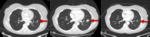
Response to cytotoxic chemotherapy on imaging is typically characterized by a prompt reduction in size of treated lesions.
This classical pattern of response is not always seen in patients responding to treatment with checkpoint inhibitors.
Several novel patterns of treatment response have been described which can make response assessment in these patients challenging.
In addition,
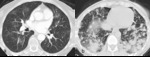
there are numerous immune-mediated side effects,
several of which have imaging correlates that can mimic disease progression and add to difficulty in determining treatment response.
Treatment response
In patients who...
Conclusion
When assessing patients receiving immunotherapy,
radiologists need to be aware of the potential for non-traditional patterns of tumor response and immune related adverse events.
Personal information
K.
Harrington,Department of Radiology,
Memorial Sloan Kettering Cancer Center,
1275 York Ave,
New York,
10065,
United States
D Halpenny,Department of Radiology,
Memorial Sloan Kettering Cancer Center,
1275 York Ave,
New York,
10065,
United States
References
1. Schadendorf,
D.,
et al.,
Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol,
2015.
33(17): p.
1889-94.
2. Huang,
J.,
et al.,
The efficacy and safety of nivolumab in previously treated advanced non-small-cell lung cancer: a meta-analysis of prospective clinical trials. Onco Targets Ther,
2016.
9: p.
5867-5874.
3. Topalian,
S.L.,
et al.,
Safety,
activity,
and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med,
2012.
366(26): p....