Aims and objectives
There is an increasing interest on recruiting imaging biomarkers for evaluating treatment effects.The potential ability of an imaging biomarker to act as an indicator of a biological process and for monitoring the response to therapy can be severely influenced by the lack of reproducibility and repeatability.
These two terms are commonly confused,
with varying degrees of consistency on their terms found in the literature.
Repeatability tests,
often named as test-retest studies,
refer to the variation in repeat measurements of the same imaging biomarker under identical...
Methods and materials
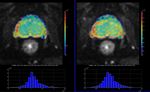
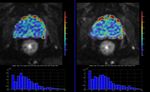
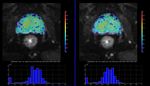
A multi-b value diffusion sequence,
optimized for the quantification of diffusion imaging biomarkers was acquired twice (test-retest scheme) in the same imaging session using a 1.5T MR Scanner (Philips,
Ingenia) in eighteen patients with locally confined prostate cancer.
The diffusion sequence comprised 7 b values (0,
50,
100,
200,
500,
1000 and 1500 s/mm2) and 4 different models including Gaussian mono-exponential (gm),
Gaussian bi-exponential (gb),
non-Gaussian mono-exponential (ngm) and non-Gaussian bi-exponential (ngb) were applied to quantify the following diffusion biomarkers: ADC,
Dgb,
D*gb,
fgb,
Dngb,...
Results
Results:All CVs were lower than 10%,
except D*gbthat was 16.55%.
More specifically,
Dngmand Dgbprovided with the lowest CV values (2.45%,
2.67%,
respectively),
followed by ADC (3.04%),
Dngb(3.62%),
fgb(4.01%),
Kngm(4.61%),
fngb(5.56%),
Kngb(5.84%) and D*ngb(9.71%).
Conclusion
Both Gaussian and non-Gaussian diffusion models offer imaging biomarkers like ADC,
D,
f,
and K (with the exception of D*) with very high repeatablity therefore longitudinal assessment of treatment induced changes based on the quantification of these diffusion biomarkers is feasible in patients with prostate cancer.
References
Abramson,
R.
G.,
K.
R.
Burton,
J.
P.
Yu,
E.
M.
Scalzetti,
T.
E.
Yankeelov,
A.
B.
Rosenkrantz,
M.
Mendiratta-Lala,
B.
J.
Bartholmai,
D.
Ganeshan,
L.
Lenchik and R.
M.
Subramaniam.
2015.
"Methods and challenges in quantitative imaging biomarker development." Acad Radiol 22(1):25-32
Waterton,
J.
C.
and L.
Pylkkanen.
2012.
"Qualification of imaging biomarkers for oncology drug development." Eur J Cancer 48(4):409-415
(ESR),
European Society of Radiology.
2013.
"ESR statement on the stepwise development of imaging biomarkers." Insights Imaging 4(2):147-15
Malyarenko,
D.,
C.
J.
Galbán,...