Examination technique
Fetal MRI becomes increasingly helpful after 22 weeks and 1,5T is the most common used field strength. It is a safe procedure, when performed without administration of contrast media. Fast images acquisition protocols minimize fetal, uterine and respiratory motion artifacts. HASTE (half-Fourier acquisition single-shot turbo spin-echo) and True-FISP (fast imaging with steady-state free precession) are examples of fast sequences that we use in our protocol, acquiring T2-weighted images.
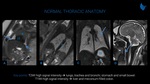
The presence of the radiologist is vital to adjust the field-of-view to the target region and to acquire images on three orthogonal planes. Sagittal sections can be achieved by placing the middle slice through the thoracic spine and the umbilical cord insertion, also coronal sections must be parallel to the thoracic spine. Regarding lung volumetry, for example, axial slices should be perpendicular to the long axis of the thoracic spine. Normal thoracic anatomy at fetal MRI is described in figure 3.
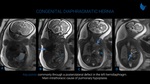
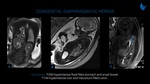
Congenital Pulmonary Airway Malformation (CPAM)
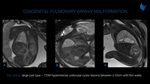
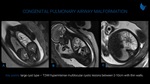
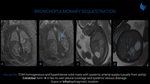
CPAMs (Fig. 4 and 5) are hamartomatous lesions that results from normal bronchoalveolar development failure with various degrees of cystic formation. There is a wide spectrum of symptoms, commonly related to recurrent infections. CPAMs can be classified into five types according to cyst size and pathologic features. Type I (large cysts, between 2-10 cm), type II (small cysts, between 0,5-2 cm) and type III (microcysts, solid appearance) are distinguishable at imaging. They have a variable appearance at T2-weighted fetal MRI that depends on the cystic and solid components. Large cysts CPAMs are unilocular or multilocular hyperintense lesions with thin walls, while microcystic lesions manifestlike a solid mass with high signal intensity. Usually, CPAM have blood supply through the pulmonary arteries and drainage through the pulmonary veins. Hybrid lesions have an abnormal systemic arterial supply, as well as CPAM and bronchopulmonary sequestration features. CPAMs may regress or have spontaneous involution (Fig. 8).
to recurrent infections. CPAMs can be classified into five types according to cyst size and pathologic features. Type I (large cysts, between 2-10 cm), type II (small cysts, between 0,5-2 cm) and type III (microcysts, solid appearance) are distinguishable at imaging. They have a variable appearance at T2-weighted fetal MRI that depends on the cystic and solid components. Large cysts CPAMs are unilocular or multilocular hyperintense lesions with thin walls, while microcystic lesions manifestlike a solid mass with high signal intensity. Usually, CPAM have blood supply through the pulmonary arteries and drainage through the pulmonary veins. Hybrid lesions have an abnormal systemic arterial supply, as well as CPAM and bronchopulmonary sequestration features. CPAMs may regress or have spontaneous involution (Fig. 8).
Bronchopulmonary sequestration
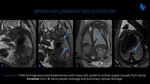
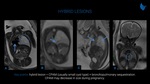
This malformation occurs when a portion of lung is originated from an additional lung bud and does not have a connection with tracheobronchial tree. Systemic arterial supply generally arises from aorta. Pleural coverage and venous drainage determine the lesion types. Intralobar lesion (Fig. 6) presents the same pleural coverage and generally drains into the pulmonary veins, while extralobar lesion (Fig. 7) does not share the same pleural investment and has systemic venous drainage. It is possible to see a solid mass with high signal intensity and its arterial vessel arising from aorta at T2-weighted images at fetal MRI. If systemic arterial supply is not identified, it may be difficult to differentiate from a microcystic CPAM.
Pulmonary hypoplasia
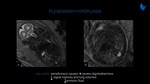
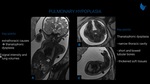
Pulmonary hypoplasia (Fig. 9 and 10) is characterized by decreased size and number of tracheobronchial structures and pulmonary vessels. It is classified as primary when no cause is identified. The most common pulmonary hypoplasia is secondary to any disease that limits the space for lung development due to a mass effect. Congenital diaphragmatic hernia is the mainintrathoracic cause and is detailed below. Extrathoracic causes include severe oligohydramnios (related to genitourinary anomalies or prolonged rupture of membranes) and hypoplastic thorax that leads to a small and rigid space for pulmonary development (e.g. thanatophoric dysplasia). Reduction of signal intensity and lung volumes are typical of T2-weighted images.
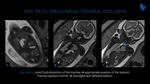
Congenital Diaphragmatic Hernia (CDH)
CDH (Fig. 11 and 12) occurs most commonly through a posterolateral defect in left hemidiaphragm musculature allowing herniation of structures (e.g. stomach, bowel or liver) into the chest. Ipsilateral and sometimes contralateral pulmonary development restriction may occur. Fetal MRI can accurately provide lung volume parameters that predict prognosis, which is related to the severity of pulmonary hypoplasia. Fetal endoluminal tracheal occlusion (FETO) can promote lung growth due to entrapment of lung fluid and may improve survival. Fluid filled structures, such as stomach and small bowel, show high signal intensity on T2-weighted images. Liver and meconium-filled colon are hyperintense in T1-weighted images.
(e.g. stomach, bowel or liver) into the chest. Ipsilateral and sometimes contralateral pulmonary development restriction may occur. Fetal MRI can accurately provide lung volume parameters that predict prognosis, which is related to the severity of pulmonary hypoplasia. Fetal endoluminal tracheal occlusion (FETO) can promote lung growth due to entrapment of lung fluid and may improve survival. Fluid filled structures, such as stomach and small bowel, show high signal intensity on T2-weighted images. Liver and meconium-filled colon are hyperintense in T1-weighted images.
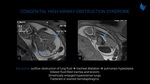
Congenital High Airway Obstruction Syndrome (CHAOS)
CHAOS (Fig. 13) is caused by fetal airway complete or almost complete outflow obstruction of lung fluid, leading to pulmonary hyperplasia and tracheal dilatation. Dilated fluid-filled trachea and bronchi helps to localize the level of obstruction. Severe heart compression may cause hydrops and ascites due to obstructed venous return. T2-weighted images showflattened or everted hemidiaphragms, dilated fluid-filled airway and enlarged hyperintense lungs. CHAOS pathophysiology explains the attempt of FETO procedure to promote lung growth in severe CDH (Fig. 14).
Esophageal Atresia
Small stomach or polyhydramnios are suspicious findings to esophageal atresia when not explained by other causes. Fluid-sensitive sequences show higher diagnostic accuracy and should be performed in patients with these findings. Normal fetuses havea characteristic high intensity stomach signal on T2-weighted images due to swallowed amniotic fluid. On the contrary, the presence of contracted stomach, polyhydramnios and esophageal pouch at fetal MRI has been shown to be highly specific in the diagnosis of esophageal atresia (Fig. 15). Finally, assessment of the type of fistulous communication is difficult to perform before birth.
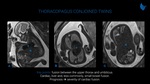
Thoracopagus Conjoined Twins
Incomplete separation of the embryonic disc in about 13-15 days post fertilization leads to conjoined twining, a complex abnormality. Thoracopagus type (Fig. 16) is characterized by the fusion between the upper thorax and umbilicus, with the fetuses sharing a common sternum, diaphragm and upper abdominal wall. They usually present cardiac, liver and, less commonly, small bowel fusion. The prognosis is determined by the severity of cardiac fusion.