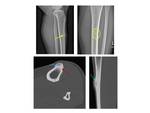
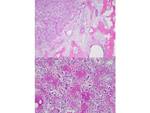
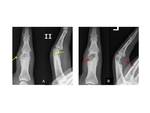
Osteoid osteoma (OO) is a benign bone producing tumor, first described by Jaffe in 1953 [1]. It accounts for 2– 3% of all bone tumors and 10–20% of benign bone tumors and is characterized radiologically by an intracortical nidus, with a variable amount of calcification, as well as cortical thickening, sclerosis, and bone marrow edema [2,3]. Fig. 1 The nidus has self-limiting growth, usually becomes asymptomatic and spontaneously heals [4,5]. Fig. 2
The typical clinical presentation of OO consists of pain that worsens at night and is promptly, but only for a short time, relieved by the administration of salicylates [6]. In physically active populations especially athletes, vague, non-specific symptoms including joint pain, swelling and mechanical symptoms coupled with non-specific radiographic findings can mimic a more common sports-related injury and delay the diagnosis of OO [7].
On the basis of computed tomography (CT) and magnetic resonance imaging (MRI) findings, Kayser et al. classified OO due to their location within the bone as follows subperiosteal, intracortical, endosteal and medullary [8].
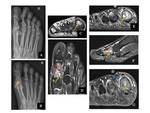
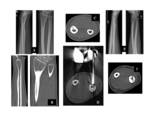
In order to detect the nidus and differentiate OO from other sclerotic lesions, high-resolution thin-section axial and longitudinal multiplanar reformatted CT images are obtained with a bone algorithm and viewed with bone window settings. Dynamic contrast-enhanced CT depicts the rapid early arterial enhancement of the nidus which appears hypervascular [2,4]. Fig. 3
MRI depicts not only the nidus and accompanying sclerosis but also adjacent bone marrow and articular abnormalities. The nidus has low to intermediate signal intensity on T1-weighted images and variable signal intensity on T2-weighted images, depending on the amount of mineralization present in the center of the nidus [2].
Although the MRI is more sensitive than a CT scan for the detection of reactive changes in soft tissue and surrounding bone edema, these can occasionally obscure the tumor itself and mimick the more aggressive lesion [9-11,12]. Fig. 4
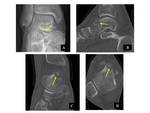
Surgical resection of OO is limited by the dissection size and the common need for cortical bone matrix transfer and internal fixation. Complications of surgical resection range at 9–28%. In addition, post-resection pain persists in 7–20% due to recurrence [13]. Fig. 5 On the other hand, CT-guided percutaneous radiofrequency ablation (RFA) is a simple, minimally invasive, safe, and highly effective technique for the treatment of OO, with reported primary success rates of 83%–94% and secondary success rates of 89%–100%. It can, therefore, be regarded as a modality of choice in most cases. Fig. 6 Open surgery should be reserved for cases of persistent diagnostic uncertainty or spinal lesions, which do not respond to medical treatment and/or can not be heated safely because of close relation to vital soft tissue structures [14].
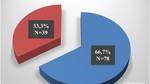
The aim of this study was to summarise epidemiological, clinical and radiological findings, and to evaluate the treatment results of patients with OO handled in our institution.