Purpose
Introduced in 2011, the Motiva SmoothSilk® is the first generation of breast implants incorporating a surface topography engineered using a 3D inverted imprinting technology to create a surface with very low roughness to avoid tissue ingrowth and minimize bacterial adhesion [4]. In addition, SmoothSilk implants use Q Inside Safety Technology™, a radio-frequency identification (RFID-M) device, located on the posterior inner surface of the implant. This identification system (micro transponder) is a passive device that emits an electronic serial number when scanned with a hand-held reader...
Methods and materials
Study design: A prospective, single-center study comprising 29 female patients who underwent breast reconstruction using Motiva SmoothSilk®(Establishment Labs Alajuela, Costa Rica) implants with RFID-M between 2018 and 2019. Breast MRI was performed two and six months postoperatively at a Breast Imaging Center in São Paulo, Brazil.
Patient population: Twenty-nine patients were submitted to breast reconstruction with SSI (total of 56 implants), as defined by having a breast cancer diagnosis and underwent partial and total mastectomy as surgical treatment. The mean age was 41.5 years (range,...
Results
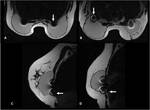
None of the patients reported local heat or discomfort during the MRI examination. There were no signs of intra or extracapsular ruptures, contractures or relevant fluid collections.
The implants' volumes ranged from 375 to 540 cm³ (average, 415 cm³) and 185 to 295 cm³ (average, 215 cm³) on the reconstructed and contralateral breast respectively.
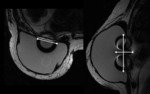
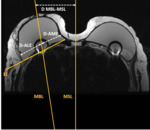
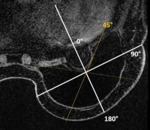
All implants showed an RFID-M related artifact with a mean estimated volume in T1 of 45.1 cm³ and in T2 of 55.6 cm³ as shown in Table 1. The mean ratio...
Conclusion
Our study, in agreement with the literature, demonstrated that the radio frequency identification (RFID-M) device contained in Motiva SmoothSilk® implants is compatible with MRI (magnetic field of 1.5T or 3.0T) [1-3].
The magnetic susceptibility artifact produced by the device was present in 100% of the cases and occurred in all MRI sequences, with the artifact volume in T1 being statistically smaller than in T2.
There was no statistically significant difference regarding the volume of the artifacts between the different surgical indications (group A and group...
Personal information and conflict of interest
M. P. F. Ananias; São Paulo/BR - nothing to disclose A. M. Munhoz; São Paulo/BR - Speaker at Establishment Labs/Motiva Implants L. F. Chala; Sao Paulo, SAO PAULO/BR - nothing to disclose G. G. N. Mello; São paulo, SÃO PAULO/BR - nothing to disclose A. G. T. D. S. Filho; São Paulo/BR - nothing to disclose H. F. B. D. Castro; SAO PAULO, SAO PAULO/BR - nothing to disclose A. D. A. M. Filho; São Paulo/BR - nothing to disclose T. C. D. M. Tucunduva;...
References
Nelson MT, Brattain KA, Williams JM. Does electronic identification enablement for silicone gel implants impact patient safety? J Surg Open Access 2018;4:1–6.
Sina Meisamy, Michael T. Nelson. The Effects of Q Inside™ Safety Technology Micro Transponder on Routine Breast Implant Imaging.Open Journal of Medical Imaging, 2019, 9, 19-31.
Fei, et al. (2014) Application Safety Evaluation of the Radio Frequency Identification Tag under Magnetic Resonance Imaging. BioMedical Engineering OnLine, 13, 129.
Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with Silksurface...