Keywords:
Thyroid / Parathyroids, Ultrasound, Biopsy, Neoplasia, Outcomes
Authors:
N. M. Popova, P. Prieditis, M. Tirane, V. Linovs, M. Rauda, K. Stepanovs, M. Radzina; Riga/LV
DOI:
10.26044/ecr2021/C-13030
Purpose
With the widespread use of imaging tests, the frequency of thyroid nodule identification has also increased. Thyroid nodules are very common in the whole population and are detected by ultrasonography in 50-60% of the population [1].
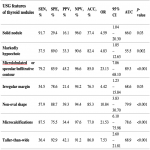
Ultrasonography currently is the best available method for the findings of nodules and its main purpose is to differentiate benign nodules from those at risk of malignancy and requiring ultrasound-guided fine-needle aspiration biopsy (FNA) to make a definitive diagnosis and decide about the necessity of surgical intervention.
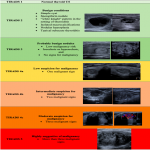
Thyroid Imaging and Reporting Data Systems (TIRADS) are crucial to patients during thyroid ultrasonography because they can help to evaluate the necessity of thyroid nodule fine-needle aspiration biopsy (FNAB). Numerous TIRADS evaluation systems have been devised in the world. Kwak et al. system, which was created in 2011, is being updated and modified version used in Latvia; however, none of the TIRADS systems is a hallmark [2].
The aim of the study is to evaluate which of the following three TIRADS systems is more sensitive and accurate: the system used in Latvia (L-TIRADS), Europe (EU-TIRADS), or the Korean TIRADS (K-TIRADS) system [2, 3, 4].